 MENU
MENU
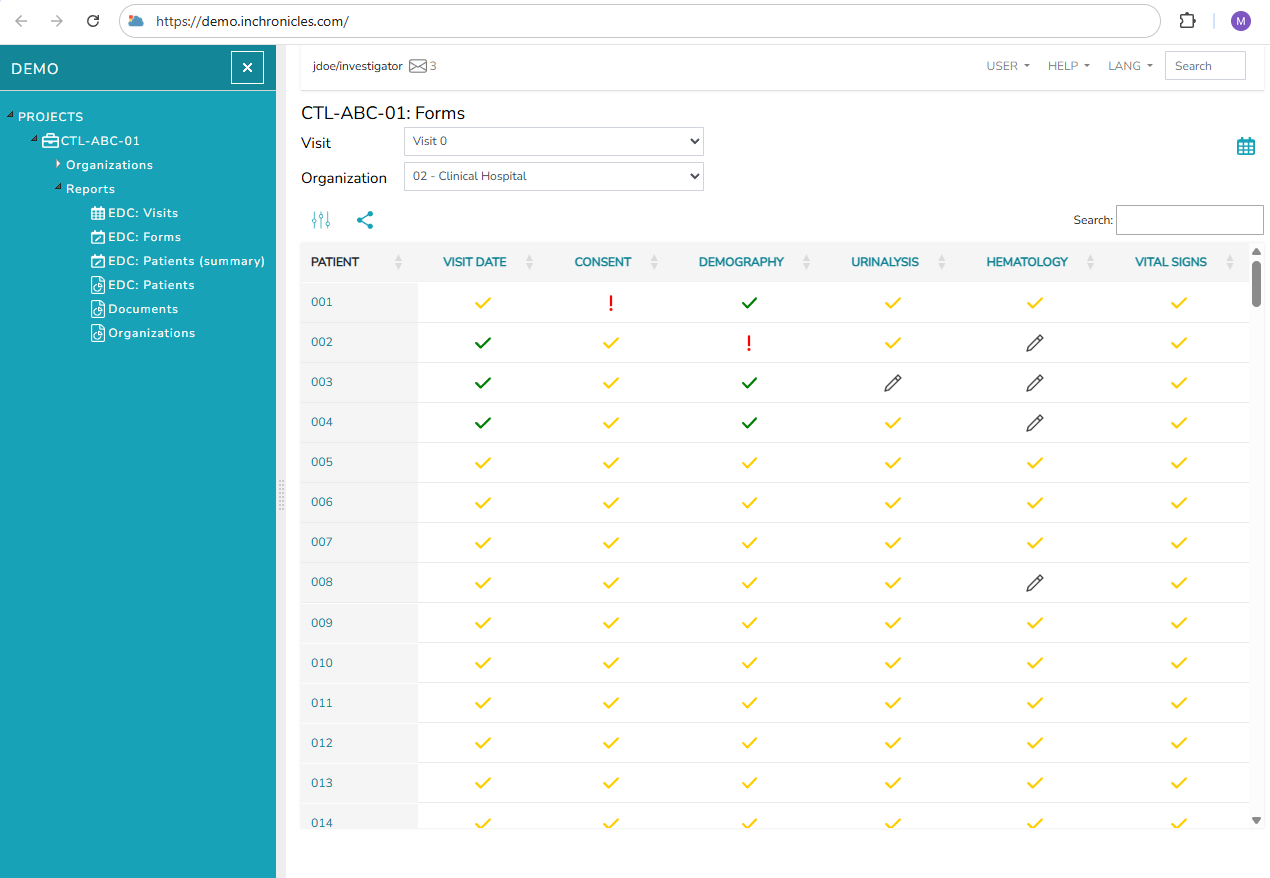
Chronicles eCRF & RTSM electronic data capture system (EDC) is the product of many years of work by our clinical research experts. The qualifications of our team include deep knowledge and wide experience in organizing, conducting, collecting and processing clinical trials data. The understanding of industry requirements is reflected in process optimization that lead to a significant reduction of time and cost of study setup in the EDC system and then during the study conduct and monitoring. That also makes a statistical analysis easier and accelerates preparation of a clinical trial report.
The Chronicles eCRF platform meets all modern approaches of data integrity and validation of computerized systems. The development and testing team strictly follows all the requirements of ICH at all stages of the software lifecycle.
Using our eCRF, you receive data which quality meets international standards.

Chronicles eCRF usage allows to setup a study within shortest timelines. For example, an initial case report forms drafting of BE study takes about five hours and not more than hour in similar projects*
* internal analytics.
We provide automation services of quality management systems starting from SOPs development and training to systems integration and business processes adapation. Our personnel have expertise in GxP and also have been involved in many research organizations audits and software development. Purchasing the system you get the full package of the documents, training and support. So you can focus on your business and delegate us all the technical issues.

We develop and support systems from the very begining to the ready-to-use product using modern agile methodology and feedback given by clients. Satisfaction of customers needs is our main concern.